BACKGROUND: Myelofibrosis (MF) is an aggressive myeloproliferative neoplasm. Abnormal growth of hematopoietic stem and progenitor cells in MF is driven by somatic mutations affecting the Janus Kinase (JAK) signaling pathway with JAK2 V617F occurring in 50-60% of patients (Cardoso et al, Plos One 2015). The role of the JAK/Signal transducer and activator of transcriptions (STAT) pathway in MF has led to the recent approval of three different JAK2 inhibitors (ruxolitinib, pacritinib, and fedratinib) for MF treatment. However, despite limiting the symptoms of the disease, JAK2 inhibitors do not result in remission due to persistence of JAK2 activation and MF-driving cells (Pandey et al, Blood Cancer J 2022). Thus, there remains a high unmet need for treatment strategies for patients with MF. Tagraxofusp, a first-in-class CD123-directed therapy, is a recombinant fusion protein consisting of human interleukin-3 conjugated to a truncated diphtheria toxin payload approved (US/EU) for the treatment of Blastic Plasmacytoid Dendritic Cell Neoplasm. In MF, CD123 is expressed on malignant cells and bone marrow (BM) accessory cells that support the proliferation of neoplastic cells (Bao et al, Hematol Oncol Stem Cell Ther 2019; Lasho et al, Blood 2014). Based on CD123 expression, tagraxofusp is a potential, novel approach for MF treatment either alone or in combination. Initial results from a two-stage multicenter, phase 2 study (STML-0401-0314; NCT02268253) of single-agent tagraxofusp in pts with relapsed or refractory (R/R) MF demonstrated encouraging clinical efficacy and a manageable safety profile (Yacoub et al. ASH 2021). We aim to further investigate the antitumoral efficacy of tagraxofusp in MF preclinical models and the potential improvement of antitumoral efficacy by combining tagraxofusp with the JAK inhibitors, ruxolitinib, and pacritinib.
METHODS: MF cell lines (SET2, UKE1, HEL: JAK2 V617F; UT7: JAK2 wild type [wt]) and bone marrow mononuclear cell (BMMC) samples from healthy donors (HD) were characterized for the extracellular expression of CD123 by flow cytometry staining. In vitro cytotoxicity experiments in CD123-positive MF cell lines (SET2, UT7, UKE-1) tested tagraxofusp and ruxolitinib or pacritinib either as single agents or in combination (MTS assay; 72 h). The combination index (CI) was calculated according to the Compusyn-Chou method (Chou TC., Pharmacol Rev, 2006) and Combenefit platform. In vitro co-culture experiments were performed with MF cell lines incubated in the presence of human primary HD-BMMCs. Cell death, as measured by LiveDead/AnnV staining, was monitored by FACS analysis through differential staining of MF cells and BMMCs with Cell Trace Violet dye (CTV). A signaling pathway analysis (JAK/STAT, S6, Mcl1) was performed through immunocapillary electrophoresis on MF cell lines treated with single agents or in combination.
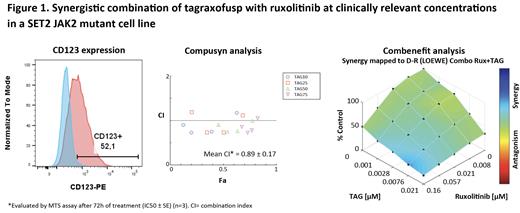
RESULTS: The MF cell lines showed different levels of CD123 expression. Except for the CD123 negative cell line (HEL), CD123 positive cell lines were all sensitive to tagraxofusp independent of CD123 expression intensity. As expected, both ruxolitinib and pacritinib were active in the presence of JAK2 wt pathway. A synergistic drug interaction was observed (mean CI range: 0.59-1.12) in most combinations of tagraxofusp with JAK inhibitors, as shown in a representative CD123+ cell line in Figure 1. When BMMCs, which are a subpopulation of BM Accessory Cells partially expressing CD123, were co-cultured with MF cell lines, the cytotoxic effect induced by tagraxofusp was enhanced, and this improvement was maintained in combination with JAK2 inhibition.
Molecular biomarkers, such as JAK/STAT, S6, and Mcl1, were evaluated in MF cell lines treated with tagraxofusp and JAK inhibitors and supported the MoA of this combination.
CONCLUSIONS: Our studies showed high sensitivity of MF cell lines to tagraxofusp as a single agent, which is expected given phase 2 results demonstrated clinical efficacy of single-agent tagraxofusp in R/R MF (Yacoub et al. ASH 2021). In addition, synergism was observed in combination with JAK inhibitors. The in vitro antitumoral effect was more pronounced in the presence of BM accessory cells, suggesting an extra targeting of CD123-positive BM cells by tagraxofusp in vivo. These results support potential clinical development of TAG in combination with JAK inhibitors for the treatment of MF.
Disclosures
Iannitto:Menarini Ricerche S.P.A: Current Employment; Nouscom: Ended employment in the past 24 months. Bisignano:Menarini Ricerche S.P.A: Current Employment. Fiascarelli:Menarini Ricerche S.P.A: Current Employment. Talucci:Menarini Ricerche S.P.A: Current Employment. Zicari:Menarini Ricerche S.P.A: Current Employment. Belli:Menarini Ricerche S.P.A: Current Employment. Bressan:Menarini Ricerche S.P.A: Current Employment. Brooks:Stemline Menarini Oncology: Current Employment. Bellarosa:Menarini Ricerche S.P.A: Current Employment. Binaschi:Menarini Ricerche S.P.A: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal